Comprehensive Quality Management Consulting Services
Achieving ISO certification can be daunting for companies operating in highly regulated industries, such as medical and aerospace. Partner with ISO-AID LLC to keep your company compliant with rigorous requirements. We provide quality management consulting services to determine areas for improvement. Contact us to schedule an audit.
Quality Management System
What Is a Quality Management System (QMS)?
A QMS is a set of policies, processes, and procedures a company uses to meet quality standards and customer requirements. A QMS typically includes a set of documented procedures that outline how the company identifies, measures, controls, and improves quality.
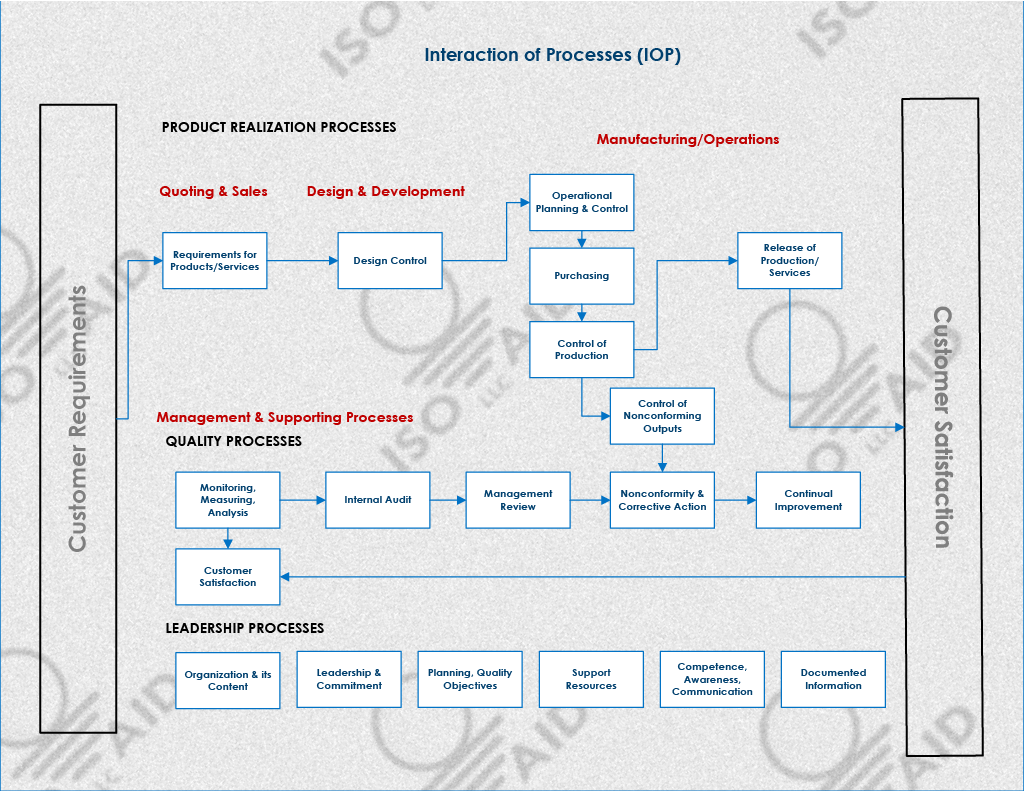
A QMS is critical for maintaining the quality and performance of a company's products and services and building customer trust and loyalty. It is regularly reviewed and improved to ensure it remains effective and relevant.
Main Components of a Quality Management System
Quality Policy:
A quality policy affirms a company's dedication to quality and outlines its approach to managing quality in its operations.
Quality Objectives:
Quality objectives are the company's targets regarding quality, such as reducing defects or improving customer satisfaction.
Quality Manual:
A quality manual details the overall structure and content of the quality management system and describes how it is implemented and maintained.
Quality Procedures:
Quality procedures outline how to perform tasks or processes consistently and in a controlled manner.
Quality Records:
Quality records are the results of quality activities, such as test results or customer complaints.
Quality Audit:
A quality audit ensures the quality management system is effective and complies with regulations and standards.
Internal Audit
What Is an Internal Audit?
An internal audit assesses a company's quality management system for compliance with ISO standards and regulations. The auditor reviews QMS aspects to determine if it operates as intended and meets quality standards.
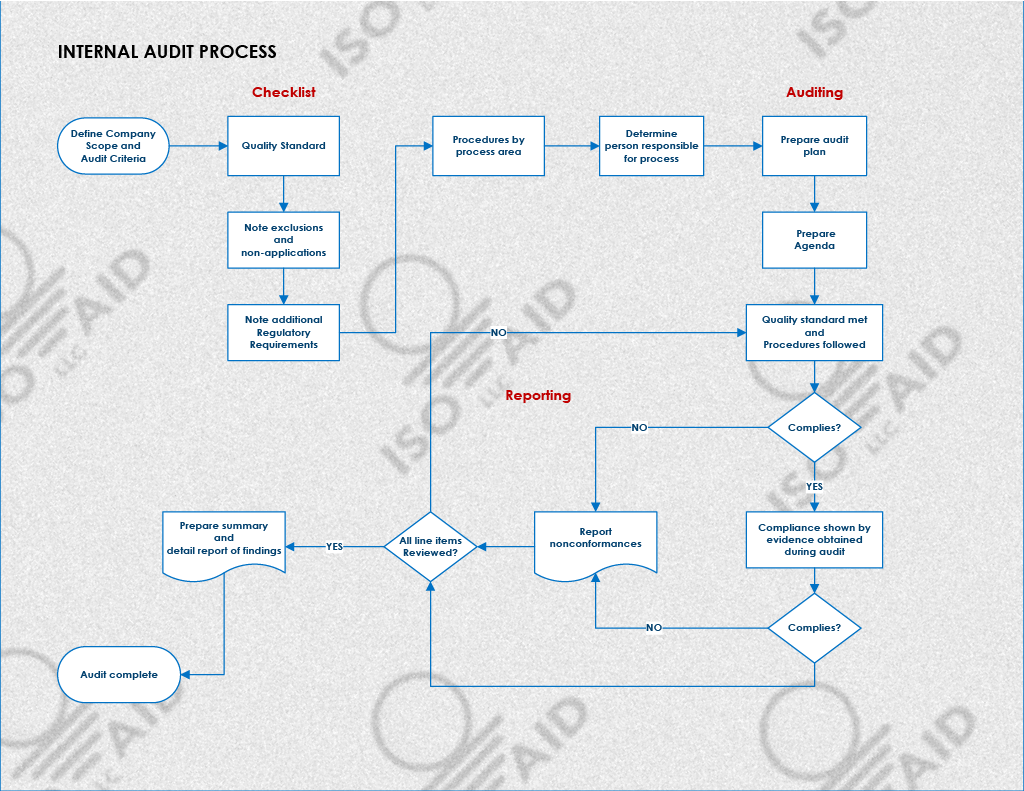
What Is Reviewed During an Internal Audit?
Areas That May Be Reviewed
Quality Policy and Objectives
Are the company's quality policy and objectives clearly defined and aligned with their business needs?
Quality Manual:
Does the company's quality manual reflect its quality management system, and is it up to date?
Quality Procedures:
Are the company's quality procedures comprehensive, up to date, and being followed consistently?
Quality Records:
Are the company's quality records accurate, complete, and maintained as per the ISO standard?
Quality Audits:
Does the company regularly and effectively conduct quality audit processes?

